Polymers—huge molecules which are made up of repeat units linked together—are the basis of all kinds of useful materials, and are found everywhere from paper, plastic, and even much of biology. When an inorganic chemist decides to try and make something polymeric, the result might look like a coordination polymer. A coordination polymer an alternating series of transition metal ion bound to a ligand with two discrete binding sites, bound to another transition ion, bound to another ligand, and so on. Coordination polymers can form 1-dimensional strands, 2-dimensional sheets, or even 3-dimensional structures.
![]()
As chemists have explored polymers and their properties, they have discovered a multitude of novel properties and uses for them. Among the most intriguing types of polymer to us in the Sazama group are conjugated polymers, especially those with conducting or semi-conducting properties. The extended conjugation of these materials, as shown below for a polymer called poly(3-hexyl)thiophene (P3HT), allows for resonance structures which translate a charge a long distance along the polymer chain. These polymers have fascinating electronic properties, and our interest in electronic structure-function relationships makes these a great inspiration to our work.
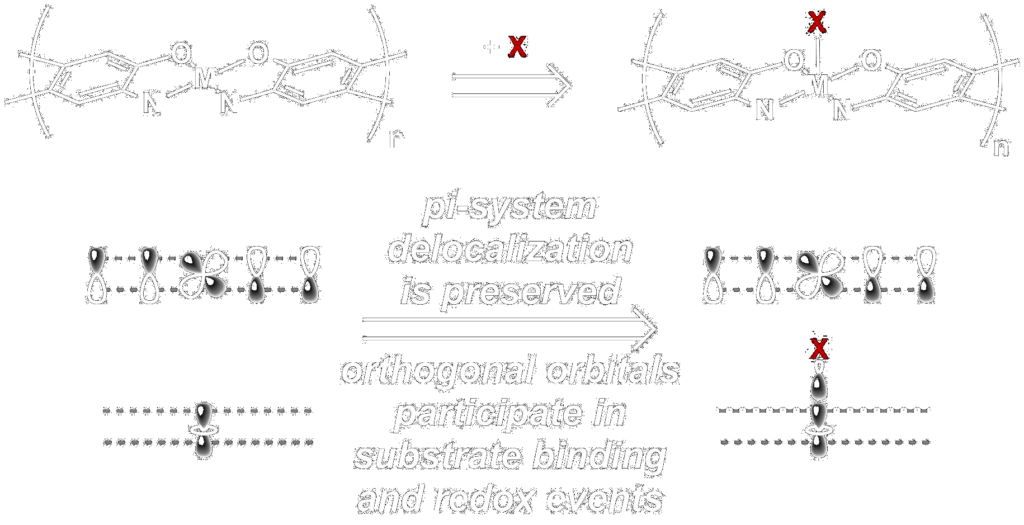
Our goal in the molecular-scale materals subgroup is to combine the worlds of coordination chemistry and conjugated polymers to make conjugated coordination polymers, with the aim of discovering new materials with conducting and semi-conducting properties. Our hypothesis is that these materials will be applicable to devices like sensors and molecular-scale electronics, due to their delocalized electronic structures in combination with the structural and chemical flexibility afforded by the transition metal d-orbitals.